gravimetric analysis or volhard method|volhard's method of titration : purchase Volhard’s method is used if the pH of the solution in the conical flask is acidic. If the solution is neutral or basic, Mohr’s method should be used. An introduction into what precipitation titration is, exploring two specific methods of precipitation titration: Mohr's and Volhard's method. Resultado da Três Gostoso Fazem Fila pra Meter no Passivo Faminto. Três Gostoso Fazem Fila pra Meter no Passivo Faminto. Apr 23rd 3. Incesto Gay - Irmãos .
{plog:ftitle_list}
webHot MILF Melissa Devassa sucking big dick and fucling like a pro in the gloryhole! Meet Melissa Devassa! 10 min Rede Sexo - 530.4k Views -. 1080p. SetSexVideos - Putaria .
what is volhard's method
is the hoae test hard
volhard's method of titration
Volhard’s method is used if the pH of the solution in the conical flask is acidic. If the solution is neutral or basic, Mohr’s method should be used. An introduction into what precipitation titration is, exploring two specific methods of precipitation titration: Mohr's and Volhard's method.This method is used when the pH of the solution after the sample has been prepared is acidic. If the pH is neutral or basic, Mohr’s method or the gravimetric method should be used. The . Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. .
When two titrants are listed (AgNO 3 and KSCN), the analysis is by a back titration; the first titrant, AgNO 3, is added in excess and the excess is titrated using the second titrant, KSCN. For those Volhard methods identified with an . Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. In some situations, .1. Volhard’s Method. Volhard’s method operates on titrating the excess titrant after the analyte’s reaction. It involves the addition of a known excess of a silver nitrate solution to precipitate chloride ions, while a potassium chromate .

This chapter describes the principles, procedures, and applications of traditional methods for analysis of minerals involving titrimetric and colorimetric procedures, ion .What is the Volhard method of analysis? Volhard process for assessing chlorine, bromine, and iodine in the form of halides by precipitating them with excess silver nitrate and using a thiocyanate solution to titrate excess.
is the hobby lobby math test hard
Visit our website: http://www.scienceready.com.auFollow our Instagram page: http://www.instagram.com/hscsciencereadyLike our Facebook page: http://www.facebo. The Volhard method is an indirect or back-titration method in which an excess of a standard solution of silver nitrate is added to a chloride-containing sample solution which is shown by the bottom arrow in Fig. 21.1.The excess silver is then back-titrated (top arrow) using a standardized solution of potassium or ammonium thiocyanate, with ferric ion as an indicator.a) Chemical analysis by gravimetric and volumetric method, and b) Atomic absorption method. The gravimetric and volumetric methods are suitable for determination of silica, barium oxide, manganese, iron, phosphorus, sulphur, alumina and other elements in manganese ores and concentrates. The atomic absorption method can be used for theGravimetric analysis and volumetric analysis are two important methods in quantitative chemical analysis. While gravimetric analysis relies on the measurement of mass and the formation of a precipitate, volumetric analysis involves the measurement of volume and the reaction between the analyte and a standardized solution.

12A-1 Properties of Precipitates and Precipitating Reagents A gravimetric precipitating agent should react specifically or at least selectively with the analyte and give precipitates that is: 1. Enough particle size for retaining on filter paper 2. High purity (free of contaminants) 3. Low solubility that no significant loss of the analyte occurs during filtrationGravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate and quantify the analyte of interest. This method is often used in environmental monitoring, pharmaceutical .What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. The principle of Gravimetric Analysis:
D.Pharma 1st Year Pharmaceutical Chemistry Chapter 2 : Volumetric and Gravimetric Analysis PDF Notes As Per New Syllabus (ER20-12T) Download. . Mohr s method, Volhard s method, Modified volhard s method, Farjan s method, Complexometric titration.Redox Titration, Redox indicator, Gravimetric Analysis, Types of Gravimetric Analysis, .
Gravimetric Analysis. Gravimetric analysis uses the mass of product (precipitate) formed through a precipitate reaction to quantify a target ion. . Volhard’s method must be performed in low pH to prevent the precipitation of Iron (III) as Fe (OH) A 3. At low H, there is relatively low amounts of hydroxide ions, so no Iron (II) Hydroxide .
Precipitation Titration | Mohr's Method | Volhard's Method | Fajan's Method | Pharmacutical AnalysisComplete Unit 3 Notes : https://imperfectpharmacy.in/Down.
Visit our website: http://www.scienceready.com.auFollow our Instagram page: http://www.instagram.com/hscsciencereadyLike our Facebook page: http://www.facebo.
Volumetric and Gravimetric Analysis, Acid Base Titration, Arrhenius theory, Bronsted Lowry theory, Lewis theory, Neutralisation Curves , Acid Base Indicators, etc, Non – Aqueous titration, Non-Aqueous Solvents, Precipitation titration, Mohr s method, Volhard s method, Modified volhard s method, Volhard * I – AgNO 3. AgNO 3 and KSCN. Fajans. Volhard \(\text{PO}_4^{3-}\) AgNO 3 and KSCN: Volhard * S 2– AgNO 3 and KSCN: Volhard * SCN – AgNO 3 and KSCN: Volhard * When two titrants are listed (AgNO 3 and KSCN), the analysis is by a back titration; the first titrant, AgNO 3, is added in excess and the excess is titrated using the .The sodium chloride content is determined by the well-known Volhard method. The sample is treated with AgNO. 3. then wet-ashed, and the excess AgNO. 3. is back titrated with KSCN. The AgNO. 3. solution must be added first, followed by the concentrated HNO. 3. This order of addition is critical to ensure complete precipitation of the chlorides . VOLHARD’S METHOD The Volhard’s method was first described by the Jacob Volhard, a German Chemist, in 1874. This is an indirect titration procedure, used for determining the anions that precipitate with silver.
If the solutions are acidic, the gravimetric method or Volhard’s method should be used. Equipment Needed burette and stand 10 and 20 mL pipettes 100 mL volumetric flask 250 mL conical flasks 10 mL and 100 mL measuring cylinders Solutions Needed Silver nitrate solution:(0.1 mol L−1) If possible, dry 5 g of
METHODS OF .cHEMICfi ANALYSIS OF FERRO-ALLOYS Methods of Chemical Analysis Sectional Committee, SMDC 2 Chtlk~ Rcprmnting DR T. BAN~BJ~~ National Metallurgical Labordory Jam&dpur ( CSIR ), SHIT K. L. BAN-rr Refractoriaa Sectional Committee, SMDC 18, IS1 DB 8. BHA~~ACEABYYA ‘Dn M.K.Boa6 Mini&y of St@, Mine4 & FuelIn the Volhard method for Ag + using KSCN as the titrant, for example, a small amount of Fe 3 + is added to the titrand’s solution. . The analysis for I – using the Volhard method requires a back titration. A typical calculation is shown in .method –Adsorption Indicators (fluorescein) Fajan’s (“Fay’yahns”) method –Fe(III) Ion Volhard method • Sensors –Potentiometric or amperometric –We will look at potentiometric sensors later. Indicators for Argentometric Titrations (Involving Ag+ions) (Ag+used as titrant or analyte) Chromate Ion: The Mohr Method Precipitation Type
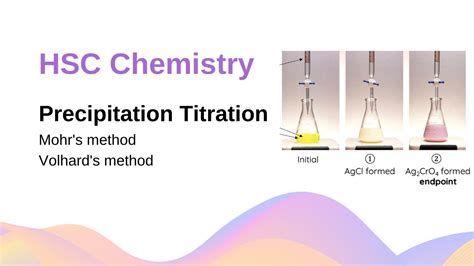
GRAVIMETRIC METHOD Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.exceeded. The appearance of red precipitate marks the endpoint. The applicability of the Mohr method is limited compared to either of the other chemical indicator methods: it can be used to analyze for Cl− or Br− anions. The Volhard Method The Volhard method of Ag+ determination is associated with argentometric titrations even though the
The sodium chloride content is determined by the well-known Volhard method. This method uses a back titration with 2 titrants like silver nitrate and potassium thiocyanate to determine the concentration of chloride ions in a solution. . Mohr's method or the gravimetric method should be used. The method is illustrated below by using the .
Volhard’s, Fajans method, estimation of sodium chloride. Complexometric titration: Classification, metal ion . and calcium gluconate. Gravimetry: Principle and steps involved in gravimetric analysis. Purity of the precipitate: co-precipitation and post precipitation, Estimation of barium sulphate. Basic Principles,methods and application of .
Discuss the significance of drying and igniting precipitates in gravimetric analysis. Explain the methods and temperatures used for drying and igniting precipitates. Difficulty: Hard. Describe the role of colored ions in the Volhard method of precipitation analysis. How does the formation of a colored secondary precipitate aid in determining .
Titration. The described approach to measuring vinegar strength was an early version of the analytical technique known as titration analysis.A typical titration analysis involves the use of a buret (Figure \(\PageIndex{1}\)) to make incremental additions of a solution containing a known concentration of some substance (the titrant) to a sample solution containing the substance . Thermogravimetry. One method for determining the products of a thermal decomposition is to monitor the sample’s mass as a function of temperature, a process called thermogravimetry.Figure 8.3.1 shows a typical thermogram in which each change in mass—each “step” in the thermogram—represents the loss of a volatile product. As the following example . 3 different types of precipitation titration 1. Volhard’s method of precipitation titration. Introduced by: Jacob Volhard Basic principle: In Volhard’s method, a standard solution of silver (Ag) ions is used to determine an unknown anion concentration in an acidic media (as we did in the titration example above).Ferric ions are used as an indicator.
Precipitation titration is a type of titration in which a precipitate is formed during the titration process. Precipitation titration refers to volumetric techniques based on the formation of a slightly soluble precipitate, while the argentometric method refers to titrations using silver nitrate (AgNO3) as a precipitating agent.In analytical chemistry, the most common use of this form of .
Resultado da 26 de set. de 2021 · From fuel temp sensor’s to full out engine failures, the depth of the 777V2 seems to be quite deep, which will be a user pleaser. Exterior Inspections and Ground Handling. Spearheading through the presentation, Roman's next subject was Exterior Inspections and Ground .
gravimetric analysis or volhard method|volhard's method of titration